Atomic force microscopy (AFM) is a widely applied scanning probe microscope (SPM) technique to obtain topographic features of sample surfaces in a height image on both insulating and conducting structures with a nanometer resolution. AFM imaging does not require complex sample preparations and can be operated in both air and liquid environments. However, when imaging soft, biological samples in physiological conditions, most SPM techniques have intrinsic shortcomings. As such, dynamic AFM modes oscillate the cantilever close to the sample surface to be sensitive to the interaction forces required to image sample topography. On soft samples, even intermittent contact between AFM tip and sample should be strictly avoided to avoid sample damage of deformation. Furthermore, in physiological conditions, which includes a liquid environment with high salt concentrations, the attractive tip-sample interaction forces required for non-contact imaging can be screened.
Here, Scanning ion conductance microscopy (SICM) is a resourceful SPM method for molecular biology and material science research, as SICM ensures non-contact imaging and works well in liquid medium with high ionic concentration. The fundamental operation of SICM relies on an ion current that flows between a nano-pipette electrode and a bath electrode. This ion current is used as feedback signal to maintain a constant distance between the pipette and the sample, allowing the nano-pipette to scan the surface for topography imaging. Most importantly, the sensitivity to changes of the ion current achieves measurements without any physical contact between pipette and sample. This aspect is essential to image soft biological samples, especially living cells.
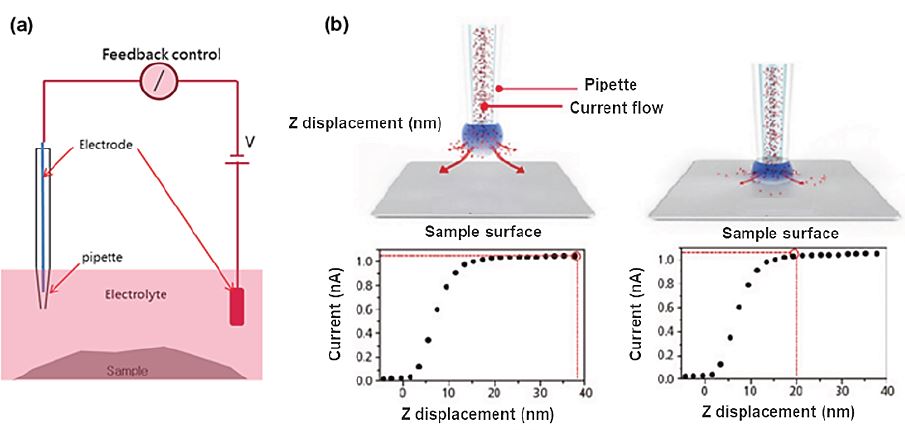
Figure 1. Illustration of the experimental setup of a Scanning ion conductance microscopy (SICM). (a) A nano-pipette filled with electrolyte is brought in proximity to a sample of interest. A bias applied between an electrode in the pipette and another electrode in the bulk solution generates an ion current, which can be used in feedback control. (b) Ion current vs. Z displacement curve at far and near pipette-sample distances.
SICM was developed by Hansma et. al. in 1989. Unlike other SPM techniques, SICM was designed specifically to non-invasively investigate biological systems. The working principle of SICM is based on monitoring changes in the ion current between two electrodes in an electrolyte solution: one inside a nano-pipette and the other in the electrolyte as shown in figure 1 (a). Usually, SICM uses silver / silver chloride (Ag/AgCl) electrodes. The voltage applied between the two electrodes across the electrolyte then introduces a current signal from the bath electrode to the pipette electrode. As the distance between pipette opening and sample decreases, there is a partial blockage of ion current, which has a distinct distance dependence as shown in figure 1 (b). While the nano-pipette is scanning the sample surface in close proximity, the feedback control detects local current signal changes and readjusts the pipette-sample distance via the Z scanner to maintain a constant current signal. From the movement of the Z scanner, we finally obtain the sample topography. For the lateral resolution of the SICM measurement, the inner diameter of the nano-pipette is decisive. Typically, the nano-pipette has an inner diameter of ~ 100 nm, but diameters of down to 30 nm are possible. The high current sensitivity of SICM ensures the absence of any physical contact between the nano-pipette and sample during the scanning and allows to record true noncontact images of the sample topography as required for soft biological samples, such as live cells.
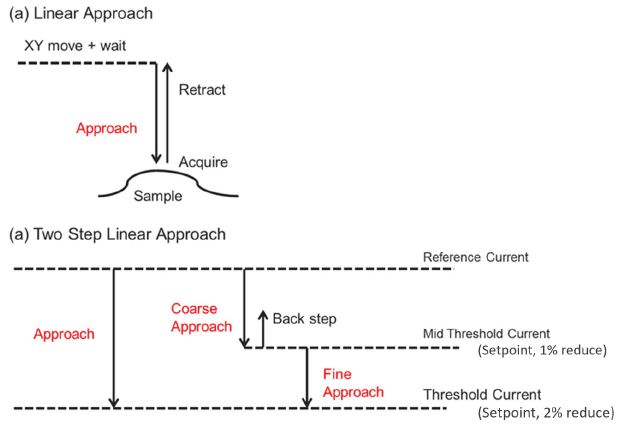
Figure 2. Operational schematic of the ARS mode. (a) Standard linear approach, (b) two-step linear approach for sensitive measurements. Retract: High value of lift height guarantees the integrity of the pipette but increases the approach time. Approach: There are two steps for approach, first a fast approach (coarse) and then a slow approach (fine). Current setpoint: based on the reference current far from the surface, maintains a constant pipette-sample distance.
There are two operation modes available for SICM on Park Systems' AFMs. One is the direct current (DC) mode and the other is the approach-retract scan (ARS) mode. In DC mode, the pipette reads the initial current value as a reference current when the pipette is far from the sample surface. From this reference current, the setpoint current for Z feedback is determined (e.g., 99% of the reference current). Subsequently, the nano-pipette approaches the sample surface until the setpoint is reached and the SICM measurement can start. During scanning, topographic features such as surface protrusions or indentations will lead to a locally decreased or increased current signal, respectively. This deviation from the current setpoint is detected as error signal and, using the error signal, the Z scanner lifts or lowers the nano-pipette until the setpoint is reestablished. In this way, the surface topography is obtained via continuous scanning at a constant pipette-sample distance.
However, imaging delicate features of biological samples or cells with height differences of several micrometers in DC mode can lead to distortions in the resulting topography. ARS mode measures the current signal by approaching and retracting the pipette from the surface at each pixel of the scan. Each approach and retract cycle first determine the reference current far from the surface and the current setpoint. Subsequently, the pipette approaches the sample surface until the current value reaches the setpoint, where the position of the Z scanner is recorded. Finally, the pipette is retracted sufficiently far from the sample to avoid pipette damage and moves on to the next imaging point. Thereby, ARS-SICM provides extremely stable imaging conditions at a lower scan rate than DC mode. For particularly sensitive measurements, ARS also offers a finer two-step approach technique, besides the regular linear approach (figure 2). The two-step approach consists of a faster coarse approach and a slow fine approach to avoid pipette or sample damage.
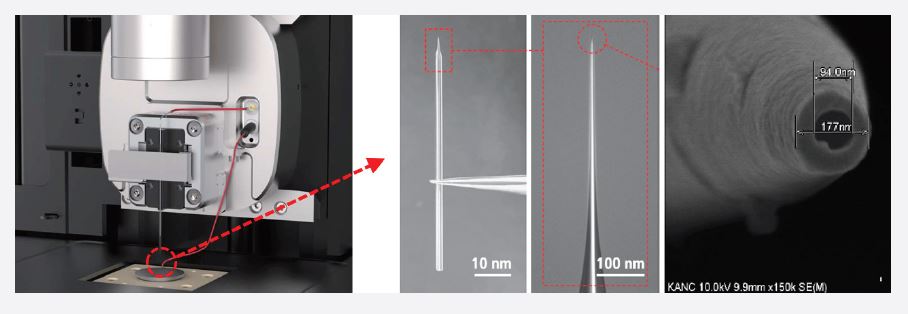
Figure 3. Park Systems’ SICM scan head with the appropriate nano-pipette positioning and all electrical connections. Optical and scanning electron microscopy (SEM) images of a nano-pipette.
Where AFM applies a cantilever with a nanoscale tip, SICM uses nano-pipettes as probes for imaging (figure 3). These nanopipettes are made from glass capillaries (inner diameter: 0.58 mm, outer diameter: 1 mm, length: 5 ~ 55 mm) or quartz capillaries (inner diameter: 0.5 ~ 50 mm, outer distance: 1.0 mm, length: 5 ~ 55 mm), which are pulled by using a pipette puller. The inner diameter of the resulting nano-pipettes is determined by the tip length, the glass capillaries’ inner diameter, the wall thickness, and the puller parameters. Usually, a 100 nm inner diameter is utilized for SICM imaging.
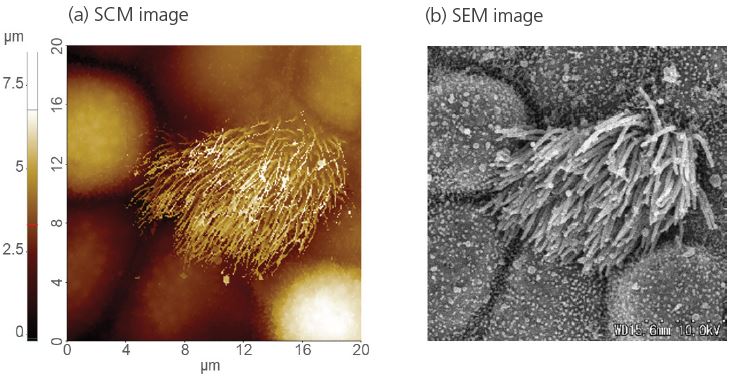
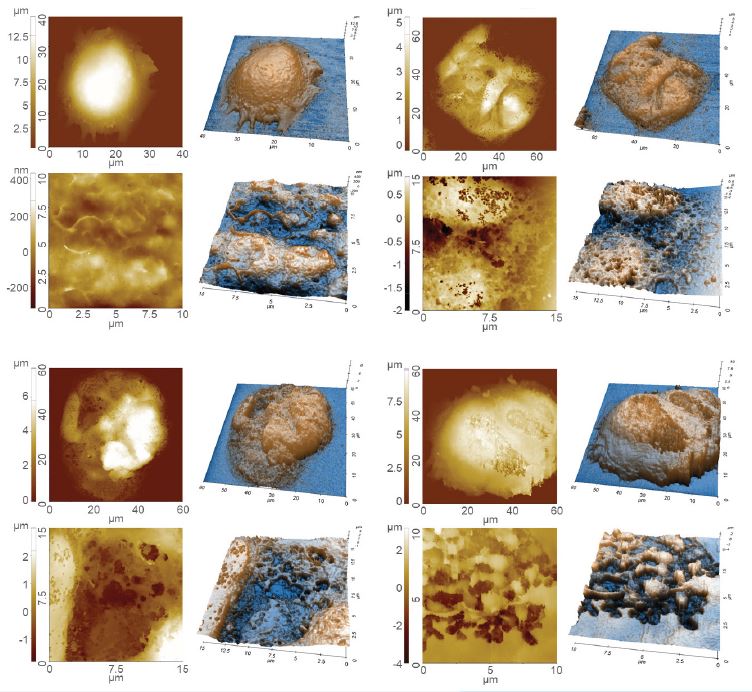
Figure 4 shows a representative measurement on a ciliated cell in tracheal tissue to highlight the potential of SICM for cell imaging. SICM was able to acquire the complicated cell morphology in high magnification and without single contact to the sample surface, which would otherwise deform the cell. As shown in the SICM image in figure 4 (a) cells can spread their cilia to surrounding cells to connect and form a complex network structure. The height changes of the structure exceed several micrometers. This measurement clearly demonstrates the unique capability of SICM to acquire the surface topography even on complicated cell structures in liquid. Figure 5 shows the topographical information of both untreated (figure 5 (a)) and detergent-treated cell surfaces (Triton X-100, 0.01%, 0.1% and 1% of concentration in figure 5 (b), (c) and (d), respectively). There are significant topography differences between untreated and detergenttreated cell surfaces, even at a small detergent concentration. Smooth and clean structures were observed on the untreated cell surface, while the detergent-treated cell membranes featured holes and surface disruptions. For this study SICM offered unique insights into the changed surface structure upon detergent treatment, by obtaining high-resolution images without inflicting damage to the cell surface. SICM is a promising tool for biological applications.
Figure 4. Ciliated cell in tracheal tissue of rat imaged by SICM in liquid condition (a) and SEM in vacuum condition (b). Image courtesy: Prof. Ushiki, Niigata University, Japan
Figure 5. Cell surface images obtained by SICM: Untreated cell (a); cell treated with 0.01% Triton X-100 (b); cell treated with 0.1% Triton X-100 (c) and a cell treated with 1% Triton X-100 treated cell (d).