AFM methodologies for quality assessment of lithium-ion battery electrodes
Introduction
Lithium ion-based batteries (LIBs) are the state-of-the-art portable energy storage to power our daily life. Ever progressing advances in both miniaturization and storage capacities made it the system of choice to be applied in smartphones, laptops or even cars. However, further advances and quality control require a deep understanding of the interplay of morphology and electronic properties on a nanometer scale. This application note briefly introduces the basic principles of batteries and the analysis of lithium-ion battery materials using atomic force microscopy (AFM).
A typical LIB consists of two electrodes, a separator, and an electrolyte solution, as depicted in Fig. 1 [1]. When electrochemical oxidation and reduction reactions occur in the electrode, ions move between the oxidation and reduction electrodes through the electrolyte, and electrons move between the two electrodes through the conducting wire at the same time. In this case, electrons move along an external conductor connecting the two electrodes, and thus a closed circuit is configured as a whole. In the anode, an electrochemical oxidation reaction of an electrode material occurs during discharge of a battery, and the electrode is called an anode. Conversely, discharge refers to the process of converting chemical energy in a battery into electrical energy. During the discharge of the battery, a reduction reaction of the electrode material occurs in the cathode by electrons transferred from the anode through an external circuit, which is called a cathode. A separator is positioned between the two electrodes to prevent an electrical short between the cathode and the anode, and electrolyte is an ion conductor that transfers ions between the two electrodes, and has no conductivity to electrons but only ion conductivity.
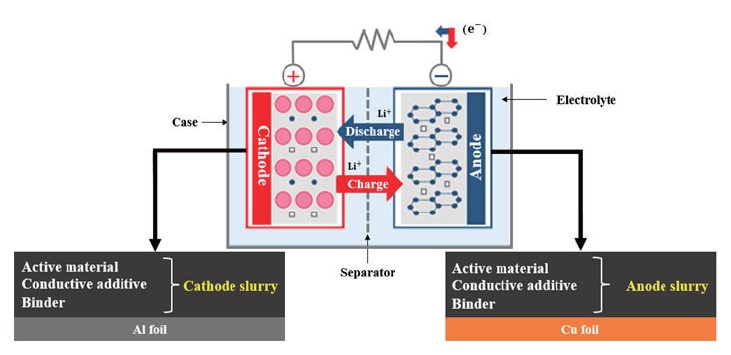
Fig. 1. Schematic of lithium-ion battery.
Electrodes for LIB typically contain active materials, conductive materials, and binders as shown in Fig. 2. The active material is responsible for generating electrical energy by reacting chemically with the anode material of the battery. An active material in a cathode material is called a 'cathode active material', and an active material in an anode material is called an 'anode active material'. The cathode active material in the cathode material has lithium ions and serves to provide lithium ions to the anode when charging the battery. The cathode active material affects battery capacity and power, and a combination of characteristics of each material such as lithium cobalt oxide (LCO), lithium manganese oxide (LMO), lithium cobalt aluminum (NCA), and lithium cobalt manganese (NCM) is used as necessary. In addition, the anode active material in the anode material generates electrical energy by storing and releasing lithium ions moving from the anode when the battery is discharged. In the anode active material, graphite, which is stable for the movement of lithium, is mainly used, and silicon, which can increase energy density, is added to improve the performance of the anode material. The conductive material is a material that promotes the movement of electrons between the cathode active material and the anode active material. It mainly uses carbon black (Super-p), and it plays an important role in connecting active materials to ensure electrical properties even in small amounts and improving battery performance. Finally, the binder is an adhesive material that helps the active material and the conductive material adhere well to the current collector. If a battery is repeatedly charged and discharged, its performance may be degraded due to volume change of anode material, such as battery life and charging time, and this problem can be supplemented by a binder that improves the binding strength of electrode materials.
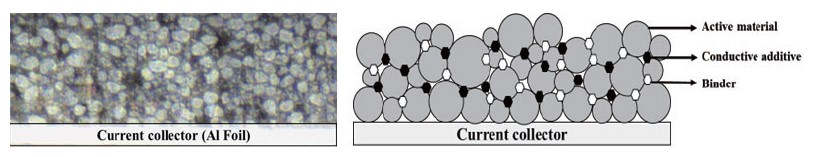
Fig. 2. Cross-sectioned pristine cathode sample (a) CCD vision image, (b) a schematic diagram
As mentioned earlier, the voltage and capacity of the battery are determined by the type of active material, and the cycling stability of LIB is directly related to the integrity of the electrode. For example, silicon was introduced as a promising anode material that can provide 10 times higher capacity than a conventional graphite anode [2]. However, silicon anodes exhibit huge volume changes during the charge-discharge processes of LIBs [3]. The stress induced by volume change can cause anode fracture and delamination. In addition, cathode materials were found to experience structural reconstruction and chemical evolution at the surface which directly influences the functionalities of cathode materials [4,5]. As a result, the formation of microcracks, porosity, and fragmented pieces leads to voltage decay and capacity loss during cycling. Therefore, it is crucial to investigate local electronic properties as well as changes to the morphology to gain a better understanding of the failure mechanism of LIBs. In this application note, we demonstrate how AFM can serve as a versatile platform to provide correlative information of the topography as well as key parameters like the work function locally.
In the past few years, various analytic methods such as scanning electron microscope (SEM), transmission electron microscope (TEM), and AFM have been intensively employed in research and development of LIBs. Although SEM and TEM measurements can provide quick results, sample analysis has limitations when using conventional electron beam-based imaging methods. Depending on the properties of interest, experiments should be carried out under a variety of conditions and parameters. Hence, a flexible and comprehensive analytical tool is required.
AFM has increasingly been used for studying electrode materials for LIBs [6–9] as it allows measuring various material properties at micro-and nano-meter spatial resolutions. In particular, cathode and anode materials for LIBs can be investigated and evaluated using the AFM to give an overview of pristine material characteristics and to help control electrode fabrication. AFM is also useful in probing mechanical and electrical degradations caused by cycling of LIBs to give an insight into the degradation mechanism of electrode materials. Surface potential measurements of electrode materials have been used to detect the degradation of electrodes. It was found that the surface potential of the cathode decreased with an increasing number of charge/discharge cycles [8]. Also, a study on the electrical degradation of cathode materials has reported a reduction in electrical conductivity along with microcracks formation within particles of the active material as the number of cycles increased [6].
Our previous study introduced Park Systems’ PinPoint™ scanning spreading resistance microscopy (SSRM) measurement using a high-vacuum AFM as an effective way to simultaneously characterize the surface, mechanical, and electrical properties of LIB electrodes [9]. In-vacuum measurements are typically used for materials that are easily oxidized or hydrolyzed in ambient conditions like cycled electrodes. However, for simplicity in instrumental and experimental setups, pristine samples can be measured under ambient conditions using conventional AFMs. This application note introduces an example of measurements on a pristine cathode as a first step into the characterization and quality assessment of LIBs.
Materials and Methods
A Ni-based layered material LiNi0.8Co0.15Al0.05O2 (NCA) is a promising cathode material for LIBs and was investigated for this application note. The cathode slurry including NCA, conductive agent, and binder was deposited onto an Al current collector. For microscopic measurements, a cross-sectional sample was prepared using an argon-ion cross-section polisher. The Al current collect part of the sample was electrically connected to the AFM’s sample bias line using silver paste. SEM measurements were conducted prior to AFM scanning to provide an overview of the structure and microscopic uniformity of the sample.
It was measured using automatic AFM [10] (FX40, Park Systems) and is equipped with advanced technology that automatically enables superluminescent diode (SLD) beam alignment and position sensitive photo diode (PSPD) alignment on the top of the cantilever. The beam was focused within the cantilever to minimize optical interference that can cause artifacts in measurement results. The automatic tip exchanger and laser aligner help reduce labor work and human error when handling the AFM tips. By employing StepScan™ function of SmartScan software, which is data acquisition software of Park Systems’ AFM, an easy setup for automated measurements, areas of interest as well as imaging modes, and scan parameters can be programmed with a simple mouse click, freeing up the operator’s time for other tasks. A conductive material coated tip (PPP-EFM, Nanosensors) was applied to measure the surface potential across the sample surface using sideband kelvin probe force microscopy (KPFM) mode. For a quantitative KPFM measurement, the work function of the AFM tip was calibrated on a freshly-cleaved HOPG surface prior to data collection on the sample. Then, SSRM measurements were conducted to measure the conductivity and resistance of the sample locally. Here, a conductive diamond-coated tip (AD-40-AS, Adama Innovations) with a nominal spring constant of 40 N/m was used to enhance the electrical signal stability and to reduce tip wear caused by lateral shear forces. Measurements were conducted at three randomly selected positions on the sample to check the uniformity of the cathode fabrication method. In addition, all AFM experiments were measured in the environmental control glove box because the uniformity could be reduced due to deformation of the sample when exposed to air.
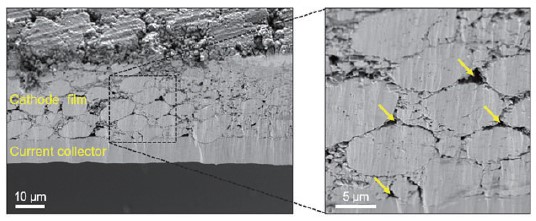
Fig. 3. SEM images obtained on cross-sectioned pristine cathode sample.
Fig. 3 presents SEM images taken on the cross-sectioned cathode sample. The cathode thickness was determined to be about 80 ± 10 μm. (The thickness of the slurry may vary depending on the loading level.) It can be clearly seen from the result that the cathode film was well-attached to the Al current collector. As shown in the data, the active material particles exhibit a relatively uniform spherical shape and high distribution density, which is expected to help enhance the electrical contact and avoid damage to the current collector during electrode fabrication. In addition, a small amount of porosity was observed (as denoted by yellow arrows), likely associated with the method of sample fabrication. However, it is expected that this small degree of porosity does not affect the overall cathode performance. Based on SEM measurements, the microscopic structure and uniformity of the electrode can be observed to provide an initial evaluation and quality control of the electrode fabrication process.
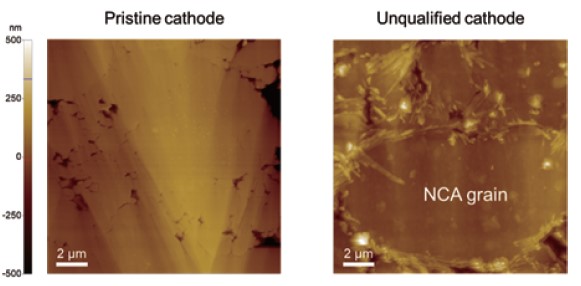
Fig. 4. Topographies of the pristine and unqualified cathode.
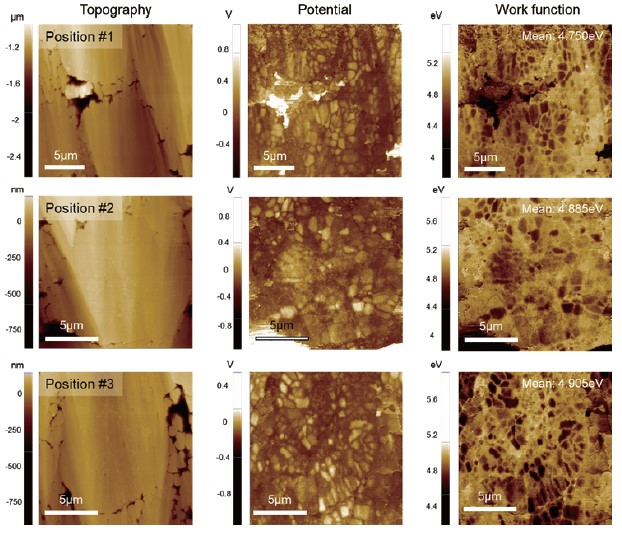
Fig. 5. KPFM results obtained at three different positions on the sample.
Surface potential measurements at different locations were summarized in Fig. 5. Since KPFM is a non-contact-based technique, a KPFM measurement yields both topography and surface potential simultaneously in a single scan. Considering that different AFM tips exhibit different work function values, a potential offset between tip and sample will vary accordingly. Therefore, to compare the surface potential between different batches of samples or between samples that are prepared and characterized at different facilities, all measurements should be conducted using the same single tip, which is sometimes not possible. For comparison, it is better to quantify the measured surface potential into a work function unit. The calibration can be performed using reference materials with a known work function such as HOPG, gold, etc. From the data in Fig. 4, the work function of the NCA particle was determined to be 5.0 ± 0.2 eV, which is in good agreement with the reported theoretical value of 4.9 eV. The results of these KPFM measurements suggest that the pristine electrode sample is in good condition and is ready to go to a battery assembly line. In addition, the comparative data in Fig. 4 shows that KPFM can reveal more details on the sample characteristics which are difficult to observe by solely inspecting the surface topography.
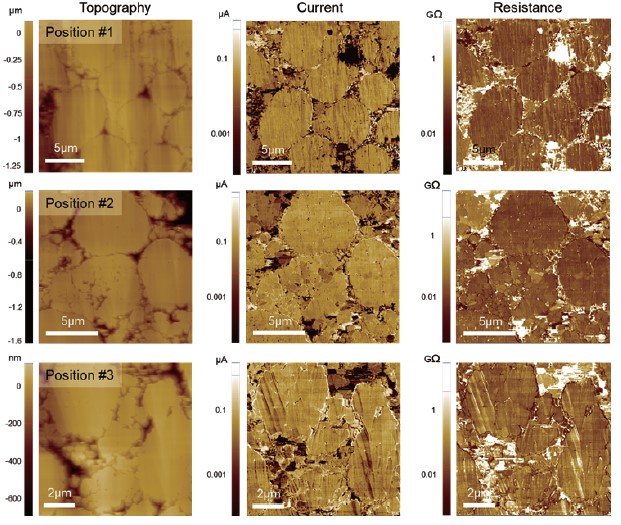
Fig. 6. SSRM results obtained at three different positions on the sample. A DC bias of 1V was applied to the current collector.
Fig. 6 shows SSRM measurements obtained on the sample. A similar level of current was observed regardless of measurement positions indicating a uniformly distributed conductivity across the cathode film. Although the applied bias to the current collector was relatively small, a sufficient amount of current was measured at the cathode film. This suggests that the prepared cathode sample exhibit high electrical conductivity which is advantageous in improving the performance of LIBs [6]. From SEM and AFM measurements, it can be concluded that the pristine cathode sample has a uniform structure and good electrical characteristics which are preferred for LIBs with prolonged lifetime. Additionally, due to the characteristics of battery samples, it is recommended to measure with PinPoint SSRM mode because the active material may be removed or broken if measured with general SSRM mode. PinPoint electrical measurements can simultaneously measure terrain, electrical and mechanical properties and prevent tip wear and sample damage by lateral forces after contact.
Conclusion
In this application note, a pristine cathode for LIBs was prepared and characterized. In particular, the structure, topographical, and electrical properties of the sample were measured and interpreted to provide a better understanding of the relationship between electrode characteristics and battery performance. The approach used in this work suggests an example of how to utilize the AFM to inspect and assess the quality of the electrodes for LIBs and therefore provide a pathway for future battery research and development. In addition to the techniques introduced in this work, various AFM modes are also available for intensive battery characterization, establishing AFM powerful tool for battery research. Such modes include:
- Conductive AFM mode to measure current distribution and conductance change of the electrode during battery cycling.
- PinPoint electrical modes to simultaneously measure the mechanical and electrical properties of the electrode.
- Electrochemical AFM for in-situ measurement of the electrode surface changes due to electrochemical processes in the battery.
- Environment control options: AFM measurements are available in different conditions such as high vacuum, in gas and liquid, and in controlled temperature and humidity conditions.
Accordingly, AFM provides a versatile platform to provide correlative information on the nanometer scale.
Reference
1 . B. Goodenough, K.-S. Park, The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc. 135 (2013) 1167–1176.
2. U. Kasavajjula, C. Wang, A.J. Appleby, Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells, J. Power Sources. 163 ( 2007) 1003–1039.
3. L.Y. Beaulieu, T.D. Hatchard, A. Bonakdarpour, M.D. Fleischauer, J.R. Dahn, Reaction of Li with Alloy Thin Films Studied by In Situ AFM, J . Electrochem. Soc. 150 (2003) A1457.
4. F. Lin, I.M. Markus, D. Nordlund, T.-C. Weng, M.D. Asta, H.L. Xin, M.M. Doeff, Surface reconstruction and chemical evolution o f stoichiometric layered cathode materials for lithium-ion batteries, Nat. Commun. 5 (2014) 3529.
5. J. Zheng, M. Gu, J. Xiao, P. Zuo, C. Wang, J.-G. Zhang, Corrosion/Fragmentation of Layered Composite Cathode and Related C apacity/Voltage Fading during Cycling Process, Nano Lett. 13 (2013) 3824–3830.
6. S.Y. Park, W.J. Baek, S.Y. Lee, J.A. Seo, Y.-S. Kang, M. Koh, S.H. Kim, Probing electrical degradation of cathode materials for lithium-ion b atteries with nanoscale resolution, Nano Energy. 49 (2018) 1–6.
7. W. Zhao, W. Song, L.-Z. Cheong, D. Wang, H. Li, F. Besenbacher, F. Huang, C. Shen, Beyond imaging: Applications of atomic force m icroscopy for the study of Lithium-ion batteries, Ultramicroscopy. 204 (2019) 34–48.
8. J. Wu, S. Yang, W. Cai, Z. Bi, G. Shang, J. Yao, Multi-characterization of LiCoO2 cathode films using advanced AFM-based techniques w ith high resolution, Sci. Rep. 7 (2017) 11164.
9. J.P. Pineda, C. Lee, B. Kim, K. Lee, Electrical and Mechanical Characterization of Li Ion Battery Electrode using PinPoint™ SSRM, M icros. Today. 28 (2020) 48–53.
10. Park Systems Corporation, A New Class of Atomic Force Microscope: FX40, the Automatic AFM
