Targeted Patch Clamping with Scanning Ion Conductance Microscopy
Myung Hoon Choi, Goo Eun Jung (Research Product Management, Research & Development of Park Systems, Seoul, Korea)
Targeted patch clamping (TPC)1 combines patch clamping with ion conductance microscopy (SICM)2 to guide the pipette to a specific patch clamping position. Patch clamping is vital to the study of excitable cells such as neurons, cardiomyocites, and muscle fiber in electrophysiology because the technique allows the researcher to examine single or multiple ion channels in those cells. But patch clamping cannot be applied to small cells or submicron-size structures on the cell surface, due to the resolution limit of the incorporated optical microscope, which is used to maneuver the patch clamp’s pipette near the cell surface. Because SICM can identify the cell surface at the submicron scale using the same patch clamping pipette, TPC overcomes the optical resolution hurdle, and greatly improves the applicability of the patch clamp and its accuracy beyond the classical technique. In this paper, live rat ventricular cardiomyocyte cells were examined with targeted patch clamping using a Park SICM. Ion channel signals were successfully recorded at a chosen Z groove location on the ventricular cardiomyocyte.
Operational Principle of Targeted Patch Clamping
The main idea of TPC is to perform patch clamping on small cellular structures using SICM for submicron-scale detection. The SICM utilizes the same pipette and electric circuit used with traditional patch clamping to detect the cell topography using Park XEP control software in the SICM imaging mode, high resolution cell topography is acquired with feedback control. Once an interesting structure for patch clamping is identified via SICM imaging, the SICM Z scanner positions the pipette on the structure. A giga-ohm seal is formed by applying suction. Ion channel recording can then be performed as in conventional patch clamping. Because the pipette approaches the cell surface in the vertical direction with nanometer scale precision, TPCimproves the probability of making a giga-ohm seal compared with the slanted approach used in conventional patch clamping3.
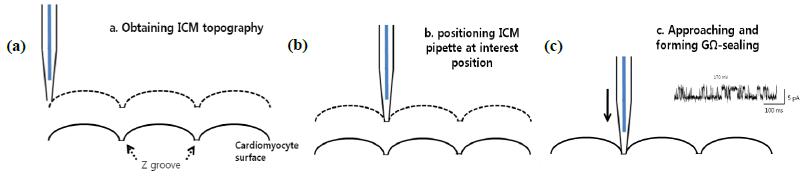
Instrumentation for Targeted Patch Clamping
To implement TPC, the SICM is integrated with a patch clamp system by sharing the pipette and its ionic current circuit. SICM receives the ionic current signal for imaging from the patch clamp’s ion current detector (pre-amp.). The ionic current flows through the nano-pipette, it is detected by the patch clamp’s amplifier, and then it is divided into two: one for the SICM cell imaging (feedback control) and the other for the electrophysiological recording.
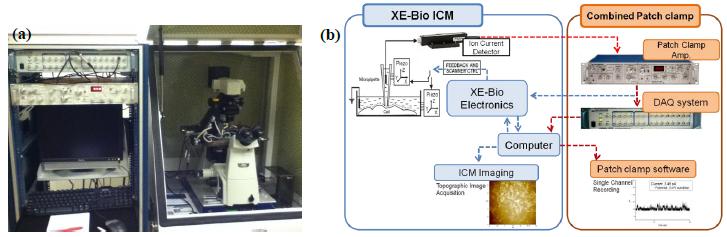
Samples and Methods
Live rat ventricular cardiomyocyte cells were isolated as previously described4. Cells were allowed to attach to polystyrene cell culture dishes filled with bath solutions composed of 120 NaCl, 5.4 KCl, 5 MgSO4, 0.2 CaCl2, 5 Na-Pyryvate, 5.5 Glucose, 20 Taurine, 10 Hepes, and 29 Manitol, adjusted to PH 7.4 with NaOH. Pipette filling solution was the same as the bath solution. A live cell image was acquired in SICM-ARS mode5. The pipette used for the SICM was pulled from borosilicate glass (O.D.,1.0 mm, I.D., 0.58 mm, length 90 mm; Warner Instruments, US) using a CO2-laser-based micropipette puller (P-2000, Sutter Instruments, Novato, US). The inner diameter of the pipette was about 500 nm.
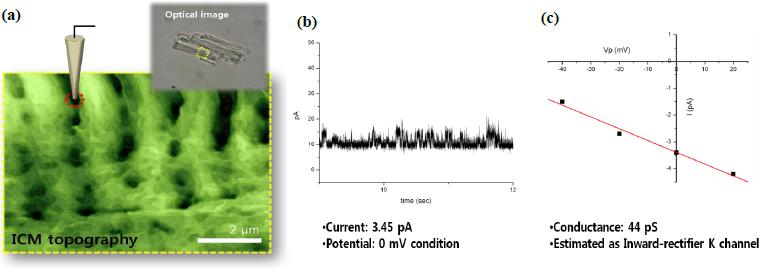
Ion Channel Recording of Targeted Patch Clamping
Figure 3 shows an example of targeted patch clamping using SICM. SICM topography illustrates typical Z grooves clearly, with details. At the position on the Z groove structure (red circle) selected from the SICM topography image, we examined the attached cell recording and successfully measured a transient current of about 3.45 pA at a voltage of ±0 mV. This result indicates that Park XE-Bio SICM is well-suited for both precise targeting and patch clamping on the cell membrane. The SICM is especially useful for research on small cells such as sperm cells, subcellular structures such as microvilli6, and even opaque cells like intact tissues, trachea, and brain slices.
References
1J. Gorelik, G. Yuchun et al. Biophys. J. 83, 3296 (2002).
2P. K. Hansma, B. Drake, O. Marti, S. A. Gould, C. B. Prater, Science 243, 641 (1989).
3O.P. Hamill, A. Marthy, E. Neher, B. Sakmann, F.J. Sigworth, J. Physiol. 391, 85 (1981)
4S.E. Harding, G. Vescovo, M. Kirby, S.M. Jones, J. Gurden, and P.A. Poole-Wilson, J.Mol. Cell. Cardiol. 20. 635 (1988).
5C. Myunghoon, XE-Bio Application Note 1, Park Systems (2012)
6G. Yuchun, G. Julia, et al. FASEB J. 16, 748 (2002)
