Principles and Application of Brewster Angle Microscopy
INTRODUCTION
Reflection of light from surfaces is part of our daily experience. However, an amazing property of optics is the possibility of having zero reflection. In more detail, no reflection occurs from a clean and perfect interface illuminated under a unique angle of incidence with p-polarized light. This well-understood phenomenon is described by Brewster´s law providing the so called Brewster’s angle for the involved optical media. Any subsequent changes of the optical properties at the interface will lead to reflection. This fact is the basis of Brewster angle microscopy, a recent technique for the study of nanofilms at the water/air interface or at the surface of other transparent nonadsorbing dielectric substrates.
Even just with a monolayer on an interface Brewster’s law is not fulfilled and the covered part of the surface reflects light, although with very low intensity. By increasing the intensity of the illumination the contrast between the covered and the clean part of the surface can be enhanced. By using a microscope optic and a CCD-detector high contrast images of the lateral morphology of the layer can be taken. Typical fields of application are life sciences, colloid and surface chemistry, and materials research.
BREWSTER’S LAW
When a light beam passes the boundary between two media of differing refractive index, generally some of it is reflected. The Brewster's angle (θB) is a particular angle of incidence where light with one particular polarization state cannot be reflected. This state is parallel to the the plane of incidence. Light with this polarization is said to be p-polarized.
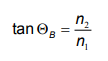
For an air-water interface (n2 = 1.33) in an ambient of air (n1 = 1), the Brewster's angle for visible light reaches approximately 53° to the normal. Since the refractive index for a given medium is a function of the wavelength of light, Brewster's angle will also vary in wavelength.
BREWSTER ANGLE MICROSCOPY
Brewster angle microscopy was introduced in 1991. No light is reflected from the air-water interface under Brewster’s angle of incidence, if p-polarized light is used. With constant angle of incidence, the formation of a monolayer on the water surface modifies the Brewster’s angle condition, and light reflection is observed.
Figure 1 illustrates the principle of obtaining the contrast for a surface covered with a nanofilm by Brewster angle microscopy.
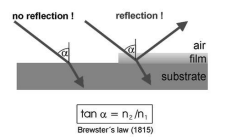
Figure 1: Principle of Brewster angle microscopy: Making use of Brewster`s Law for imaging ultra-thin films with high contrast.
Brewster angle microscopy is an effective visualisation method for substructures with long range orientational order. To obtain a contrast between subdomains of different molecule orientations an analyzer is positioned in the reflected beam path.
INSTRUMENTATION
The origin of commercial Brewster angle microscopes was the instrument developed by Dirk Hönig in his diploma-thesis (figure 3). Since the first presentation at the LB6 in Paris Brewster angle microscopy has been established as a worldwide standard technique for the investigation of ultra-thin films. Currently we offer two different Brewster angle microscopes. The nanofilm_ep4bam (figure 2) is based on the ellipsometric platform nanofilm_ep3 and can be upgraded to an imaging ellipsometer.
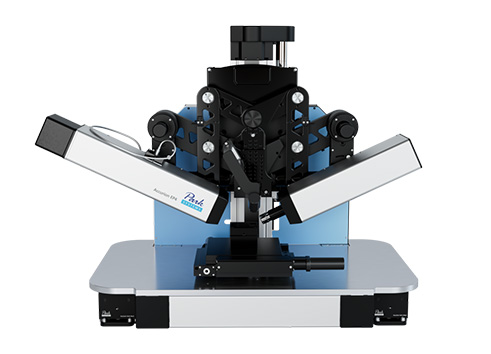
Figure 2: nanofilm_ep4bam with ultraobjective
The new nanofilm_ultrabam (figure 3) is based on a complete new optical pathway of light. The unique imaging optic provides fully focused images at max. 35 frames per second. Thus, for the first time in a commercial instrument high resolution AND overall focused real time imaging of monolayers becomes possible. It enables the visualization of Langmuir monolayers or adsorbed films in real time.
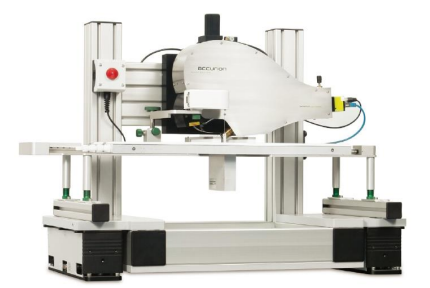
Figure 3: nanofilm_ultrabam
APPLICATIONS
Morphological features of monolayers during compression/decompression
The morphological study of monolayers has led to a more detailed understanding of the two-dimensional condensed phase structure of monolayers. Brewster angle microscopy can be used for direct observations during compression of monolayers in a Langmuir trough. The advantage against epi fluoresence and AFM is that no markers are required and that the film does not need to be transferred to a solid substrate.
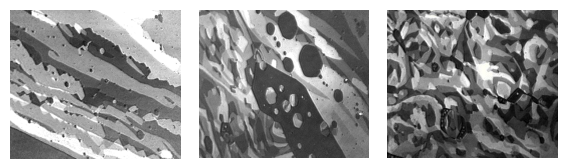
Figure 4: Ethyl stearate monolayer at ƍ<1mN/m, Field of view ca. 600μm (nanofilm_ultrabam)
Inner structure of 2D condensed phase domains
As mentioned, Brewster angle microscopy is an effective method to vizualize the substructure with longrange orientational order by using an analyzer. Typical examples are methyl esters of fatty acids.
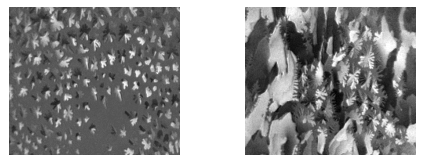
Figure 5: Monolayer of DMPE during first-order phase transition, contrast in domains caused by long range orientation order (nanofilm_ultrabam).
Formation dynamics, non equilibrium structures
The formation of the condensed phase in a fluid monolayer can occur far from equilibrium, and growth kinetics can be so fast that the stable phase does not have time to reach its lowest energy state on the microscopic level. Metastable microstructures are formed under non-equilibrium conditions and the growth patterns of these structures are mainly affected by the complex interplay of microscopic interfacial dynamics and external driving forces.
Monolayers & chirality
Monolayers of enantiomers of amphiphilic molecules can differ. The interaction of enantiomers and diastereomer pairs can be investigated for specific monolayer states. Figure 6 shows domains of D-Dipalmitoylphosphatidylcholin (D-DPPC), L-DPPC and the racemate.
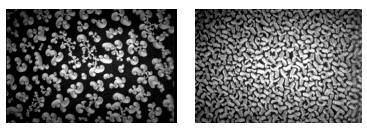
Figure 6: Brewster angle micrograph of of D-Dipalmi-toylphosphatidylcholin and the racemate
INTEGRATION OF OTHER TECHNIQUES
The information obtained with the Brewster angle microscopy can be complemented by other optical techniques like imaging ellipsometry or UV/VIS reflection spectroscopy. With imaging ellipsometry, it could be possible to e.g. determine the optical parameters of two phases of a monolayer observed by BAM, like domains and surrounding areas. With UV/VIS reflection spectroscopy it is possible to e.g. observe absorption bands in the monolayers and their growth or possible shift, which can provide valuable information about the molecular interactions happening in the monolayer, like the formation of domains or multilayers.
